CARISOPRODOL AND ASPIRIN
-
carisoprodol and
aspirin tablet
Rising Pharmaceuticals, Inc.
CIV
Rx only
Carisoprodol and Aspirin tablets, USP are a fixed-dose combination product containing the following two products:
It is available as a two-layered, Red and White, round tablet for oral administration.
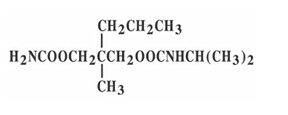
Carisoprodol: Chemically, carisoprodol is N-isopropyl-2-methyl-2-propyl-1,3propanediol dicarbamate and its molecular formula is C12H24N2O4, with a molecular weight of 260.33. The structural formula of carisoprodol is:
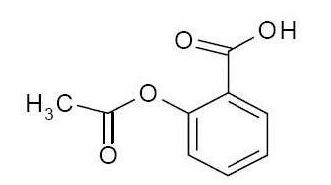
Aspirin: Chemically, aspirin (acetylsalicylic acid) is 2-(acetyloxy-, benzoic acid and its molecular formula is C9H8O4, with a molecular weight of 180.16. The structural formula of aspirin is:
Other ingredients in the Carisoprodol ·and Aspirin Tablets, drug product are Com starch, FD&C Red # 40 Aluminum Lake, Hydroxypropyl Cellulose, Lactose Anhydrous, Microcrystalline Cellulose, Magnesium Stearate, Pregelatinized Starch, Sodium Starch Glycolate and Sodium Lauryl Sulfate.
Carisoprodol: The mechanism of action of carisoprodol in relieving discomfort associated with acute painful musculoskeletal conditions has not been clearly identified. In animal studies, muscle relaxation induced by carisoprodol is associated with altered interneuronal activity in the spinal cord and in the descending reticular formation of the brain.
Aspirin: The mechanism of action of aspirin in relieving pain is by inhibition of the body's production of prostaglandins, which are thought to cause pain sensations by stimulating muscle contractions and dilating blood vessels.
Carisoprodol: Carisoprodol is a centrally-acting muscle relaxant that does not directly relax tense skeletal muscles. A metabolite of carisoprodol, meprobamate, has anxiolytic and sedative properties. The degree to which these properties of meprobamate contribute to the safety and efficacy of Carisoprodol and Aspirin is unknown.
Aspirin: Aspirin is a non-narcotic analgesic with anti-inflammatory and anti-pyretic activity. Inhibition of prostaglandin biosynthesis appears to account for most of its anti-inflammatory and for at least part of its analgesic and antipyretic properties. In the CNS, aspirin works on the hypothalamus heat-regulating center to reduce fever. Aspirin can cause serious gastrointestinal injury including bleeding, obstruction, and perforations from ulcers possibly by inhibition of the production of prostaglandins, compromising the defenses of the gastric mucosa and the activity of substances involved in tissue repair and ulcer healing (see WARNINGS). Aspirin inhibits platelet aggregation by irreversibly inhibiting prostaglandin cycle-oxygenase. This effect lasts for the life of the platelet and prevents the formation of the platelet aggregating factor thromboxane A2.
Carisoprodol: The pharmacokinetics of carisoprodol and its metabolite meprobamate were studied in a study of 24 healthy subjects (12 male and 12 female) who received single doses of 350 mg of carisoprodol (see Table 1). The Cmax of meprobamate was 2.5 ± 0.5 μg/mL (mean ± SD) after administration of a single 350 mg dose of carisoprodol, which is approximately 30% of the Cmax of meprobamate (approximately 8 μg/mL) after administration of a single 400 mg dose of meprobamate.
Table 1: Pharmacokinetic Parameters of Carisoprodol and Meprobamate (Mean ± SD, n=24)
| Carisoprodol | Meprobamate | |
| Cmax (μg/mL) | 1.8 ± 1.0 | 2.5 ± 0.5 |
| AUCinf (μg·hour/mL) | 7.0 ± 5.0 | 46 ± 9.0 |
| Tmax (hour) | 1.7 ± 0.8 | 4.5 ± 1.9 |
| T1/2 (hour) | 2.0 ± 0.5 | 9.6 ± 1.5 |
Absorption: Absolute bioavailability of carisoprodol has not been determined. After administration of a single dose of 350 mg of carisoprodol, the mean time to peak plasma concentrations (Tmax) of carisoprodol was approximately 1.5 to 2 hours. Co-administration of a high-fat meal with 350 mg of carisoprodol had no effect on the pharmacokinetics of carisoprodol.
Metabolism: The major pathway of carisoprodol metabolism is via the liver by cytochrome enzyme CYP2C19 to form meprobamate. This enzyme exhibits genetic polymorphism (see Patients with Reduced CYP2C19 Activity below).
Elimination: Carisoprodol is eliminated by both renal and non-renal routes with a terminal elimination half-life of approximately 2 hours after administration of a single dose of 350 mg of carisoprodol. The half-life of meprobamate is approximately 10 hours after administration of a single dose of 350 mg of carisoprodol.
Gender: Exposure of carisoprodol is higher in females than in male subjects (approximately 30 to 50% on a weight adjusted basis). Overall exposure of meprobamate is comparable between female and male subjects.
Patients with Reduced CYP2C19 Activity: Carisoprodol should be used with caution in patients with reduced CYP2C19 activity. Published studies indicate that patients who are poor CYP2C19 metabolizers have a 4-fold increase in exposure to carisoprodol, and 50% reduced exposure to meprobamate compared to normal CYP2C19 metabolizers. The prevalence of poor metabolizers in Caucasians and African Americans is approximately 3 to 5% and in Asians is approximately 15 to 20%.
Aspirin:
Absorption: The rate of aspirin absorption from the gastrointestinal (GI) tract is dependent upon the presence or absence of food, gastric pH (the presence or absence of GI antacids), and other physiologic factors. Following absorption, aspirin is hydrolyzed to salicylic acid in the gut wall and during first-pass metabolism with peak plasma levels of salicylic acid occurring within 1 to 2 hours of dosing.
Distribution: Salicylic acid is widely distributed to all tissues and fluids in the body including the central nervous system (CNS), breast milk, and fetal tissues. The highest concentrations are found in the plasma, liver, kidneys, heart, and lungs. The protein binding of salicylate is concentration dependent, i.e., nonlinear. At plasma concentrations of salicylic acid < 100 μg/mL and > 400 μg/mL, approximately 90 and 76 percent of plasma salicylate is bound to albumin, respectively.
Metabolism: Aspirin, which has a half-life of about 15 minutes, is hydrolyzed in the plasma to salicylic acid such that plasma levels of aspirin may not be detectable 1 to 2 hours after dosing. Salicylic acid, which has a plasma half life of approximately 6 hours, is conjugated in the liver to form salicyluric acid, salicyl phenolic glucuronide, salicyl acyl glucuronide, gentisic acid, and gentisuric acid. At higher serum concentrations of salicylic acid, the total clearance of salicylic acid decreases due to the limited ability of the liver to form both salicyluric acid and phenolic glucuronide. Following toxic doses of aspirin (e.g., > 10 grams), the plasma half-life of salicylic acid may be increased to over 20 hours.
Elimination: The elimination of salicylic acid is constant in relation to the plasma salicylic acid concentration. Following therapeutic doses of aspirin, approximately 75, 10, 10, and 5 percent is found excreted in the urine as salicyluric acid, salicylic acid, a phenolic glucuronide of salicylic acid, and an acyl glucuronide of salicylic acid, respectively. As the urinary pH rises above 6.5, the renal clearance of free salicylate increases from less than 5 percent to greater than 80 percent.
Alkalinization of the urine is a key concept in the management of salicylate overdose (see OVERDOSAGE, Treatment of Overdosage). Clearance of salicylic acid is also reduced in patients with renal impairment.
Carisoprodol and Aspirin is indicated for the relief of discomfort associated with acute, painful musculoskeletal conditions in adults. Carisoprodol and Aspirin should only be used for short periods (up to two or three weeks) because adequate evidence of effectiveness for more prolonged use has not been established and because acute, painful musculoskeletal conditions are generally of short duration (see DOSAGE AND ADMINISTRATION).
Carisoprodol and Aspirin is contraindicated in patients with a history of:
Carisoprodol has sedative properties and may impair the mental and/or physical abilities required for the performance of potentially hazardous tasks such as driving a motor vehicle or operating machinery. There have been post-marketing reports of motor vehicle accidents associated with the use of carisoprodol.
Since the sedative effects of carisoprodol and other CNS depressants (e.g., alcohol, benzodiazepines, opioids, tricylic antidepressants) may be additive, appropriate caution should be exercised with patients who take more than one of these CNS depressants simultaneously.
In post-marketing experience with carisoprodol, cases of dependence, withdrawal, and abuse have been reported with prolonged use. Most cases of dependence, withdrawal, and abuse occurred in patients who have had a history of addiction or who used carisoprodol in combination with other drugs with abuse potential. However, there have been post-marketing adverse event reports of carisoprodol-associated abuse when used without other drugs with abuse potential. Withdrawal symptoms have been reported following abrupt cessation after prolonged use. To reduce the chance of carisoprodol dependence, withdrawal, or abuse, carisoprodol should be used with caution in addiction prone patients and in patients taking other CNS depressants including alcohol, and carisoprodol should not be used more than two to three weeks for the relief of acute musculoskeletal discomfort.
Carisoprodol, and one of its metabolites, meprobamate (a controlled substance), may cause dependence (see CLINICAL PHARMACOLOGY).
Aspirin can cause serious gastrointestinal (GI) adverse reactions including bleeding, perforation, and obstruction of the stomach, small intestine, or large intestine, which can be fatal. Aspirinassociated serious Gl adverse reactions can occur anywhere along the Gl tract, at any time, with or without warning symptoms. Patients at higher risk of aspirin-associated serious upper Gl adverse reactions include patients with a history of aspirin-associated Gl bleeding from ulcers (complicated ulcers), a history of aspirin-associated ulcers (uncomplicated ulcers), geriatric patients, patients with poor baseline health status, patients taking higher doses of aspirin, and patients taking concomitant anticoagulants, NSAIDs, and/or large amounts of alcohol. To minimize the risk for aspirin-associated Gl serious adverse reaction, the lowest effective aspirin dose should be used for the shortest possible duration.
Aspirin may cause an increased risk of serious anaphylaxis and anaphylactoid reactions, which can occur in patients without known prior exposure to aspirin (see CONTRAINDICATIONS). Patients with a serious anaphylaxis or anaphylactoid reaction should receive emergency care.
The safety and pharmacokinetics of Carisoprodol and Aspirin in patients with renal or hepatic impairment have not been evaluated.
Since carisoprodol is excreted by the kidney and is metabolized in the liver, caution should be exercised if carisoprodol is administered to patients with impaired renal or hepatic function. Carisoprodol is dialyzable by hemodialysis and peritoneal dialysis.
There have been postmarketing reports of seizures in patients who received carisoprodol. Most of these cases have occurred in the setting of multiple drug overdoses (including drugs of abuse, illegal drugs, and alcohol) (see OVERDOSAGE).
Gastrointestinal Adverse Reactions
In addition to serious gastrointestinal adverse reactions, the use of aspirin is also associated with gastritis, gastrointestinal erosions, abdominal pain, heartburn, vomiting, and nausea (see WARNINGS, Serious Gastrointestinal Adverse Reactions).
Patients should be advised to contact their health care provider if they experience any adverse reactions to Carisoprodol and Aspirin Tablets.
Aspirin:
Carisoprodol:
The sedative effect of carisoprodol and other CNS depressants (e.g., alcohol, benzodiazepines, opioids, tricyclic antidepressants) may be additive. Therefore, caution should be exercised with patients who take more than one of these CNS depressants simultaneously. Concomitant use of carisoprodol and meprobamate, a metabolite of carisoprodol, is not recommended (see WARNINGS, Sedation).
Carisoprodol is metabolized in the liver by CYP2C19 to form meprobamate (see CLINICAL PHARMACOLOGY). Co-administration of CYP2C19 inhibitors, such as omeprazole or fluvoxamine, with carisoprodol could result in increased exposure of carisoprodol and decreased exposure of meprobamate. Co-administration of CYP2C19 inducers, such as rifampin or St. John’s Wort, with carisoprodol could result in decreased exposure of carisoprodol and increased exposure of meprobamate. Low dose aspirin also showed an induction effect of CYP2C19. The full pharmacological impact of these potential alterations of exposures in terms of either efficacy or safety of carisoprodol is unknown.
Aspirin: Clinically important interactions may occur when certain drugs or alcohol are administered concomitantly with aspirin.
Alcohol: Concomitant use of aspirin with ≥ 3 alcoholic drinks may increase the risk of GI bleeding (see WARNINGS, Serious Gastrointestinal Adverse Reactions).
Anticoagulants: Concomitant use of aspirin and anticoagulants (e.g., heparin, warfarin, clopidogrel) increase the risk of GI bleeding (see WARNINGS, Serious Gastrointestinal Adverse Reactions). Additionally, aspirin can displace warfarin from protein binding sites, leading to prolongation of the international normalized ratio (INR).
Antihypertensives: The concomitant administration of aspirin with angiotensin converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), beta-blockers, and diuretics may diminish the hypotensive effects of these anti-hypertensive products due to aspirin’s inhibition of renal prostaglandins, which may lead to decreased renal blood flow and increased sodium and fluid retention. Concomitant use of aspirin and acetazolamide can lead to high serum concentrations of acetazolamide due to competition at the renal tubule for secretion.
Corticosteroids: Concomitant administration of aspirin and corticosteroids may decrease salicylate plasma levels.
Methotrexate: Aspirin may enhance the toxicity of methotrexate due to displacement of methotrexate from its plasma protein binding sites and/or reduction of the renal clearance of methotrexate.
Nonsteroidal anti-inflammatory drugs (NSAIDs): The concurrent use of aspirin with selective and nonselective NSAIDs increases the risk of serious GI adverse reactions (see WARNINGS, Serious Gastrointestinal Adverse Reactions).
Oral Hypoglycemics Agents: Aspirin may increase the serum glucose lowering action of insulin and sulfonylureas leading to hypoglycemia.
Products that effect urinary pH: Ammonium chloride and other drugs that acidify the urine can elevate plasma salicylate concentrations. In contrast, antacids, by alkalinizing the urine, may decrease plasma salicylate concentrations.
Uricosuric Agents: Salicylates antagonize the uricosuric action of probenecid and sulfinpyrazone.
No long-term studies of carcinogens have been done with carisoprodol and Aspirin.
Carisoprodol: Long term studies in animals have not been performed to evaluate the carcinogenic potential of carisoprodol.
Carisoprodol was not formally evaluated for genotoxicity. In published studies, carisoprodol was mutagenic in the in vitro mouse lymphoma cell assay in the absence of metabolizing enzymes, but was not mutagenic in the presence of metabolizing enzymes. Carisoprodol was clastogenic in the in vitro chromosomal aberration assay using Chinese hamster ovary cells with or without the presence of metabolizing enzymes. Other types of genotoxic tests resulted in negative findings. Carisoprodol was not mutagenic in the Ames reverse mutation assay using S. typhimurium strains with or without metabolizing enzymes, and was not clastogenic in an in vivo mouse micronucleus assay of circulating blood cells.
Carisoprodol was not formally evaluated for effects on fertility. Published reproductive studies of carisoprodol in mice found no alteration in fertility although an alteration in reproductive cycles characterized by a greater time spent in estrus was observed at a carisoprodol dose of 1200 mg/kg/ day. In a 13-week toxicology study that did not determine fertility, mouse testes weight and sperm motility were reduced at a dose of 1200 mg/kg/ day. In both studies, the no effect level was 750 mg/kg/day, corresponding to approximately 2.6 times the human equivalent dosage of 350 mg four times a day, based on a body surface area comparison.
The significance of these findings for human fertility is not known.
Aspirin: Administration of aspirin for 68 weeks in the feed of rats was not carcinogenic. In the Ames Salmonella assay, aspirin was not mutagenic; however, aspirin did induce chromosome aberrations in cultured human fibroblasts. Aspirin has been shown to inhibit ovulation in rats (see Pregnancy).
Pregnancy Category D.
It is not known whether Carisoprodol and Aspirin can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Adequate animal reproduction studies have not been conducted with Carisoprodol and Aspirin. Carisoprodol and Aspirin should be given to a pregnant woman only if clearly needed.
Carisoprodol: There are no data on the use of carisoprodol during human pregnancy. Animal studies indicate that carisoprodol crosses the placenta and results in adverse effects on fetal growth and postnatal survival. The primary metabolite of carisoprodol, meprobamate, is an approved anxiolyic. Retrospective, postmarketing studies do not show a consistent association between maternal use of meprobamate and an increased risk for particular congenital malformations.
Teratogenic effects: Animal studies have not adequately evaluated the teratogenic effects of carisoprodol. There was no increase in the incidence of congenital malformations noted in the reproductive studies in rats, rabbits, and mice treated with meprobamate. Retrospective, postmarketing studies of meprobamate during human pregnancy were equivocal for demonstrating an increased risk of congenital malformations following the first trimester exposure. Across studies that indicated an increased risk, the types of malformations were inconsistent.
Nonteratogenic effects: In animal studies, carisoprodol reduced fetal weights, postnatal weight gain, and postnatal survival at maternal doses equivalent to 1 to 1.5 times the human dose (based on a body surface area comparison). Rats exposed to meprobamate in-utero showed behavioral alterations that persisted into adulthood. For children exposed to meprobamate in-utero, one study found no adverse effects on mental or motor development or IQ scores. Carisoprodol should be used during pregnancy only if the potential benefit justifies the risk to the fetus.
Teratogenic effects: Prior to 30 weeks gestation, aspirin should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Starting at 30 weeks gestation, aspirin should be avoided by pregnant women as premature closure of the fetal ductus arteriosus which may result in fetal pulmonary hypertension and fetal death. Salicylate products have also been associated with alterations in maternal and neonatal hemostasis mechanisms, decreased birth weight, increased incidence of intracranial hemorrhage in premature infants, stillbirths, and neonatal death. Studies in rodents have show salicylates to be teratogenic when given in early gestation, and embryocidal when given in later gestation in doses considerably greater than usual therapeutic doses in humans.
Carisoprodol: There is no information about the effects of carisoprodol on the mother and the fetus during labor and delivery.
Aspirin: Ingestion of aspirin within one week of delivery or during labor may prolong delivery or lead to excessive blood loss in the mother, fetus, or neonate. Prolonged labor due to prostaglandin inhibition has been reported with aspirin use.
Carisoprodol: Very limited data in humans show that carisoprodol is present in breast milk and may reach concentrations two to four times the maternal plasma concentrations. In one case report, a breast-fed infant received about 4 to 6% of the maternal daily dose though breast milk and experienced no adverse effects. However, milk production was inadequate and the baby was supplemented with formula. In lactation studies in mice, female pup survival and pup weight at weaning was decreased. This information suggests that maternal use of carisoprodol may lead to reduced or less effective infant feeding (due to sedation) and/or decreased milk production. Caution should be exercised when carisoprodol is administered to a nursing woman.
Aspirin: Nursing mothers should avoid the use of aspirin because salicylate is excreted in breast milk which may lead to bleeding in the infant.
The efficacy, safety, and pharmacokinetics of carisoprodol and Aspirin in pediatric patients less than 16 years of age have not been established.
The efficacy, safety, and pharmacokinetics of Carisoprodol and Aspirin in patients over 65 years of age have not been established.
To report SUSPECTED ADVERSE REACTIONS, contact Rising Pharmaceuticals at 1-201-961-9000 or FDA at 1-800-FDA-1088 or www. fda.gov/medwatch.
The following adverse reactions which have occurred with the administration of the individual products alone may also occur with the use of carisoprodol and Aspirin tablets. The following events have been reported during post-approval individual use of carisoprodol and aspirin. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Carisoprodol: The following events have been reported during post-approval use of carisoprodol. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Cardiovascular: Tachycardia, postural hypotension, and facial flushing (see OVERDOSAGE).
Central Nervous System: Drowsiness, dizziness, vertigo, ataxia, tremor, agitation, irritability, headache, depressive reactions, syncope, insomnia, and seizures (see OVERDOSAGE).
Gastrointestinal: Nausea, vomiting, and epigastric discomfort.
Hematologic: Leukopenia, pancytopenia.
Aspirin: The most common adverse reactions associated with the use of aspirin have been gastrointestinal, including abdominal pain, anorexia, nausea, vomiting, gastritis, and occult bleeding (see WARNINGS, Serious Gastrointestinal Adverse Reactions and PRECAUTIONS, Gastrointestinal Adverse Reactions). Other adverse reactions associated with the use of aspirin include elevated liver enzymes, rash, pruritus, purpura, intracranial hemorrhage, interstitial nephritis, acute renal failure, and tinnitus. Tinnitus may be a symptom of high serum salicylate levels (see OVERDOSAGE).
Carisoprodol is not a Controlled Substance (see WARNINGS).
Discontinuation of carisoprodol in animals or in humans after chronic administration can produce withdrawal signs, and there are published case reports of human carisoprodol dependence.
In vitro studies demonstrate that Carisoprodol elicits barbiturate-like effects. Animal behavioral studies indicate that carisoprodol produces rewarding effects. Monkeys self administer carisoprodol. Drug discrimination studies using rats indicate that carisoprodol has positive reinforcing and discriminating effects similar to barbital, meprobamate, and chlordiazepoxide.
Signs and Symptoms: Any of the following signs and symptoms which have been reported with overdose of the individual products may occur with overdose of carisoprodol and Aspirin and may be modified to a varying degree by the effects of the other products present in carisoprodol and Aspirin.
Carisoprodol: Overdosage of Carisoprodol commonly produces CNS depression. Death, coma, respiratory depression, hypotension, seizures, delirium, hallucinations, dystonic reactions, nystagmus, blurred vision, mydriasis, euphoria, muscular incoordination, rigidity, and/or headache have been reported with Carisoprodol overdosage. Many of the Carisoprodol overdoses have occurred in the setting of multiple drug overdoses (including drugs of abuse, illegal drugs, and alcohol). The effects of an overdose of carisoprodol and other CNS depressants (e.g., alcohol, benzodiazepines, opioids, tricyclic antidepressants) can be additive even when one of the drugs has been taken in the recommended dosage. Fatal accidental and nonaccidental overdoses of Carisoprodol have been reported alone or in combination with CNS depressants.
Aspirin: Salicylate toxicity may result from an overdose of an acute ingestion or chronic intoxication. Mild to moderate salicylate poisoning is usually associated with plasma salicylic concentrations about 200 μg/mL and is characterized by tinnitus, hearing difficulty, headache, dim vision, dizziness, tachypnea, increased thirst, nausea, vomiting, sweating, and diarrhea. In the early stages of overdose, CNS stimulation and respiratory alkalosis can occur; however, in the later stages CNS depression and metabolic acidosis can occur.
Symptoms and signs of severe salicylate poisoning, associated with plasma salicylic concentrations greater that 400 μg/mL, include hyperthermia, dehydration, delirium, GI hemorrhage, pulmonary edema, and CNS depression (e.g., coma). Death is usually due to respiratory failure or cardiovascular collapse.
Overdose of aspirin in pediatric patients: Salicylate poisoning should be considered in pediatric patients with symptoms of vomiting, hyperpnea, and hyperthermia. Salicylate poisoning should be considered in infants with metabolic acidosis and all pediatric patients with severe salicylate poisoning.
Treatment of Overdosage: Provide symptomatic and supportive treatment, as indicated. For more information on the management of an overdose of Carisoprodol and Aspirin tablets, USP contact a Poison Control Center.
Carisoprodol: Basic life support measures should be instituted as dictated by the clinical presentation of the carisoprodol overdose. Induced emesis is not recommended due to the risk of CNS and respiratory depression, which may increase the risk of aspiration pneumonia. Gastric lavage should be considered soon after ingestion (within one hour). Circulatory support should be administered with volume infusion and vasopressor agents if needed. Seizures should be treated with intravenous benzodiazepines and the reoccurrence of seizures may be treated with phenobarbital. In cases of severe CNS depression, airway protective reflexes may be compromised and tracheal intubation should be considered for airway protection and respiratory support. The following types of treatment have been used successfully with an overdose of meprobamate, a metabolite of carisoprodol: activated charcoal (oral or via nasogastric tube), forced diuresis, peritoneal dialysis, and hemodialysis (carisoprodol is also dialyzable). Careful monitoring of urinary output is necessary and overhydration should be avoided. Observe for possible relapse due to incomplete gastric emptying and delayed absorption
Aspirin: Since there are no specific antidotes for salicylate poisoning, the aim of the treatment is to enhance elimination of salicylate; reduce further salicylate absorption; correct fluid, electrolyte, or acid/ibase imbalances; and provide cardio-respiratory support. The acidbase status should be followed closely with serial serum pH determinations (using arterial blood gas). If acidosis is present, intravenous sodium bicarbonate should be given, along with adequate hydration, until salicylate levels decrease to within the therapeutic range. To enhance elimination, forced diuresis and alkalinization of the urine may be beneficial. Gastric emptying and/or lavage are recommended as soon as possible after ingestion, even if the patient has vomited spontaneously. After lavage and/or emesis, administration of activated charcoal is beneficial, if less than 3 hours have passed since ingestion. Charcoal absorption should not be employed prior to emesis and lavage. In patients with renal insufficiency or in cases of life-threatening aspirin intoxication, hemodialysis or peritoneal dialysis is usually required.
Additional treatment of aspirin overdose in pediatric patients: Pediatric patients should be sponged with tepid water. Infusion of glucose may be required to control hypoglycemia. Exchange transfusion may be indicated in infants and young children.
The recommended dose of Carisoprodol and Aspirin tablets, is 1 or 2 tablets, four times daily in adults . One Carisoprodol and Aspirin tablet contains 200 mg of Carisoprodol and 325 mg of Aspirin. The maximum daily dose (i.e., two tablets taken four times daily) will provide 1600 mg of carisoprodol and 2600 mg of aspirin per day. The recommended maximum duration of Carisoprodol and Aspirin tablets use is up to two or three weeks.
Carisoprodol and Aspirin Tablets, USP 200mg/325mg are red and white, round unscored convex, two layered tablets debossed on red layer with "CL" over "023" and plain on the white layer. The tablets are available in:
Bottles of 100 NDC 64980-175-01.
Bottles of 500 NDC 64980-175-05.
Storage: Store at controlled room temperature 20° - 25°C (68° - 77°F). Protect from moisture.
Dispense in a tight container.
To report SUSPECTED ADVERSE REACTIONS, contact Rising Pharmaceuticals at 1-201-961-9000 or FDA at 1-800-FDA-1 088 orwww.fda.gov/medwatch.
Manufactured for:
Rising Pharmaceuticals, Inc.
Allendale, NJ 07401
Manufactured by:
Mirror Pharmaceuticals, LLC
Fairfield, NJ 07004 USA
5507
Rev 02/12
Rising® NDC 64980-175-01 CIV
Carisoprodol and
Aspirin Tablets, USP
200 mg/325 mg
100 Tablets
Rx Only

|
CARISOPRODOL AND ASPIRIN
carisoprodol and aspirin tablet | ||||||||||||||||||||||
| ||||||||||||||||||||||
| ||||||||||||||||||||||
| ||||||||||||||||||||||
| ||||||||||||||||||||||
| ||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| ANDA | ANDA040832 | 01/07/2022 | |
| Labeler - Rising Pharmaceuticals, Inc. (041241766) |
| Registrant - Mirror Pharmaceuticals, LLC (964680206) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Mirror Pharmaceuticals, LLC | 964680206 | MANUFACTURE | |