ACETAMINOPHEN AND DIPHENHYDRAMINE HYDROCHLORIDE
-
acetaminophen and
diphenhydramine hydrochloride tablet, film coated
HIMPRIT PHARMACHEM PVT LTD
Drug Facts
| Active ingredients (in each Caplet) | Purpose |
|---|---|
| Acetaminophen 500 mg | Pain reliever/ fever reducer |
| Diphenhydramine HCL 25 mg | Sleep Aid |
Temporary relief of occasional headaches and minor aches and pain with accompanying sleeplessness
If you consume 3 or more alcoholic drinks every day, ask your doctor if you should take acetaminophen or other pain relievers/fever reducers. Acetaminophen may cause liver damage.
Ask a doctor before use if you have
ASK a doctor or pharmacists before use if you are taking sedatives or tranquilizers
When using this product
Stop use and ask a doctor if
If pregnant or breast-feeding, ask a health professional before use
Keep out of reach of children. In case of accidental overdose get medical help or contact a Poison Control Center right away. Prompt medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
croscarmellose sodium, hypromellose, polythlene glycol,sodium metabisulfate, stearic acid,, sodium starch glycolate,collodial silicon dioxide, FD & C blue # 1
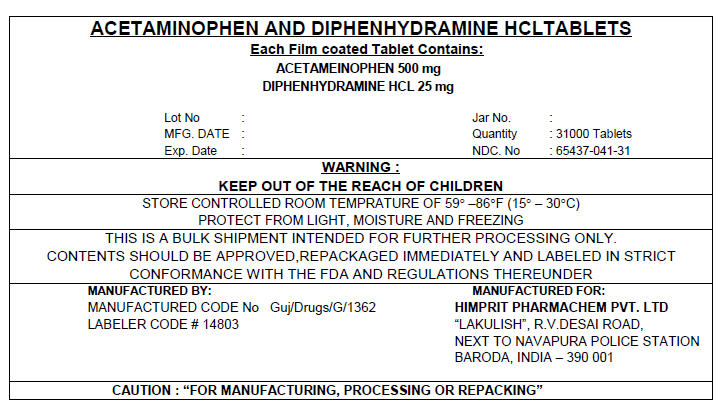
ACETAMINOPHEN AND DIPHENHYDRAMINE HCL TABLETS
Each Film coated Tablet Contains:
ACETAMEINOPHEN 500 mg
DIPHENHYDRAMINE HCL 25 mg
Lot No :
MFG. DATE :
Exp. Date :
Jar No. :
Quantity : 31000 Tablets
NDC. No : 65437-041-31
WARNING :
KEEP OUT OF THE REACH OF CHILDREN
STORE CONTROLLED ROOM TEMPRATURE OF 59° –86°F (15° – 30°C)
PROTECT FROM LIGHT, MOISTURE AND FREEZING
THIS IS A BULK SHIPMENT INTENDED FOR FURTHER PROCESSING ONLY.
CONTENTS SHOULD BE APPROVED,REPACKAGED IMMEDIATELY AND LABELED IN STRICT
CONFORMANCE WITH THE FDA AND REGULATIONS THEREUNDER
MANUFACTURED BY:
MANUFACTURED CODE No Guj/Drugs/G/1362
LABELER CODE # 14803
MANUFACTURED FOR:
HIMPRIT PHARMACHEM PVT. LTD
"LAKULISH", R.V.DESAI ROAD,
NEXT TO NAVAPURA POLICE STATION
BARODA, INDIA – 390 001
CAUTION : "FOR MANUFACTURING, PROCESSING OR REPACKING"
|
ACETAMINOPHEN AND DIPHENHYDRAMINE HYDROCHLORIDE
acetaminophen and diphenhydramine hydrochloride tablet, film coated | ||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC MONOGRAPH NOT FINAL | part343 | 07/01/2023 | |
| Labeler - HIMPRIT PHARMACHEM PVT LTD (917261992) |